Introduction
The most important technique that chemists use to determine the structures of organic compounds is Nuclear Magnetic Resonance (NMR) spectroscopy. The power of NMR spectroscopy is that it not only makes it possible to identify the positions of nuclei of interest, but also identifies the neighboring nuclei (Solomons & Fryhle, 2003). In an NMR spectrum a specific type of atom in a compound is identified by the chemical shift of its signal (the location of the signal in the spectrum) and its multiplicity (the number of peaks into which each signal is split). The multiplicity provides information about the neighboring atoms. A spectroscopy course that includes NMR spectroscopy is required for undergraduate chemistry students. However, NMR spectroscopy is often described as difficult and intangible (Dorneich & Jones, 1997) and many students have difficulty understanding NMR concepts and principles.
The problems encountered in an NMR course are summarized below.
- NMR spectroscopy requires basic concepts in mathematics, physics, and chemistry. If students lack these basic concepts, they will have difficulty understanding and visualizing NMR spectroscopy.
- Students lack skills for self-learning and solving NMR problems and may not have access to adequate textbooks or other supporting materials.
An interactive NMR spectroscopy (iNMR) coursewas developed to address these problems. Through a variety of meaningful animations and interactive assignments with relevant feedback, students can easily visualize and understand the concepts of NMR spectroscopy.
Literature Survey
Constructivist Learning Environments
Constructivism is a learning theory that offers a basis for understanding how people learn (Nussbaum, 1989; Glasersfeld, 1992). It provides researchers a theoretical framework to study how people engage with a learning object, extract relevant information from the object, and incorporate new information into their existing knowledge (Bodner, Klobuchar & Geelan, 2001).
The effectiveness of different types of learning environments may be enhanced when appropriate pedagogies are used. Constructivist learning environments are highly advocated by science educators and widely implemented in various disciplines (Tsai, 2000).Constructivist learning is often used for online learning environments and has been found to support the development of higher-order cognitive skills in organic chemistry students (Zoller & Pushkin, 2007). However, many instructional design models do not provide effective strategies for designing constructivist learning environments that allow students to take control of their learning (Lefoe, 1998). Therefore, those models may not be fully effective. Wilson and Lowry (2000) introduced three core principles for the effective use of online learning tools (simulations, animations, and websites) that provide a framework for analyzing how constructivist learning occurs in the online learning tools: 1) provide access to rich sources of information, 2) encourage meaningful interactions with content, and 3) bring people together to challenge, support, or respond to one another.
Some Relevant Websites for Learning NMR Spectroscopy
Several NMR spectroscopy websites have been developed and are available on the internet to support student learning. Some websites provide a variety of spectra useful for practicing the interpretation of NMR spectra. However, some websites do not include either the solutions for the problems or instruction in how to interpret chemical shift and multiplicity; therefore, they are useful only for students who have already learned the theory of NMR spectroscopy. On the other hand, WebSpectra: Problems in NMR and IR Spectroscopy (Merlic, Fam & Miller, 2001) and Organic Structure Elucidation: A Workbook of Unknowns(Smith, Boggess & Zajicek, 1998) are comprehensive training sites for chemistry students. NMR and IR spectra of unknowns are provided with solutions on these sites.
The following NMR spectroscopy websites support the development of problem-solving skills. First is a Virtual Textbook of Organic Chemistrywhich was developed by Reusch (1999) at Michigan State University. It contains not only multiplicity and chemical shift concepts but also solutions to problems. However, this website provides only a few problems, without spectra, and no guidance on how to interpret NMR spectra. Second is Organic Chemistry Online, developed by Young (1997) at University of Illinois at Chicago. This site consists of problems with explanations. Students can click on each signal to obtain the explanation. However, these two websites do not provide either explanations of the solutions or the concepts of chemical shift and multiplicity; nor do they explain how to interpret NMR spectra.
To improve the theoretical background of NMR, the iNMR course was developed. This website was designed to explain the concepts of NMR phenomena, processes in NMR, chemical shift, multiplicity, and NMR spectra interpretation, together with 13C-NMR and 2D-NMR, in order to help students to review their knowledge or to learn on their own before practicing problem solving. Moreover, step-by-step problem solving was designed to help the beginner. Students are challenged to solve problems with increasing levels of difficulties. When they finish with each problem set, their answers can be checked and their reasoning compared with the explanation provided.
The Development of the iNMR Course
An interactive NMR spectroscopy course was designed to provide a constructivist learning environment in which learners can engage with rich source of useful information, actively construct knowledge, and reflect on their thinking. Therefore, sub-environments in the course were designed to promote meaningful self-learning experiences (Chin & Williams, 2006 ; Wilson & Lowry, 2000 ). The planning cycle of the course is shown in Figure 1.
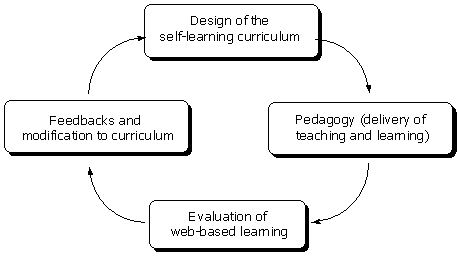
Figure 1. The planning cycle for an iNMR course (adapted from McKimm,
Jollie & Cantillon, 2003)
Self-Learning Curriculum
The NMR course contains a number of interactive animations and simulations to attract students’ interest in NMR spectroscopy. All pictures and animations, which were created by the developers, were simplified to explain principles and processes in NMR spectroscopy and to help students visualize and understand the basic concepts of NMR spectroscopy. This iNMR course emphasizes three concepts of NMR spectroscopy: chemical shift, multiplicity, and NMR spectrum interpretation or NMR problem solving. Following are descriptions of the main parts of the course.
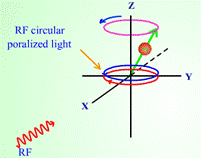
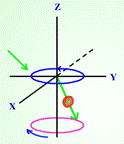
In Introduction to NMR spectroscopy, students are introduced to the NMR process and what kinds of information can be obtained from the spectrum.
|
|
a) |
b) |
Figure 2. Screen captured images showing precession of nuclear spin (a) before and
(b) after radio frequency is absorbed in a nuclear magnetic resonance process
In Chemical Shift and factors affecting chemical shift, students learn the concept of chemical shift and factors influencing chemical shift such as electronegativities of nearby atoms, hybridization of the adjacent atoms, and diamagnetic induction effects from p bonds (as shown in Figure 3).
Figure 3. Diamagnetic induction effects from p bond in a) ethylene, b) acetylene, and c) benzene
In NMR spectrum interpretation (also called NMR problem solving), students learn how to integrate the information learned in the previous sections to determine the structure of a compound. A step-by-step problem solving procedure is used to guide the beginning students to start interpreting an NMR spectrum.
Exercises, Assignments and Problem Sets
This iNMR course contains a number of interactive exercises, assignments, and problem sets. There are various types of exercises, such as multiple choice, matching, signal assignment, spectrum interpretation as well as spectrum prediction are also designed to support the development of student learning by providing pertinent feedback.
Another type of exercise is to construct the spectrum that corresponds to a given structural formula. Figure 4 illustrates the procedure involved in the spectrum prediction test. Students learn how to create a spectrum by using the concept of number of non-equivalent protons, chemical shift, multiplicity and integration. They integrate all information provided and drag the signals to compose the NMR spectrum with the chemical shift that corresponds correctly to the provided compound. After finishing the proposed spectrum (brown lines), students click on the “CHECK” button and the correct spectrum of the compound appears (blue lines in Figure 4). Each signal receives a score based on the accuracy of multiplicity, height, and chemical shift.
To encourage interest in problem solving, some of the exercises were designed as games, such as Millionaire Game. In this game, problems are arranged in four levels of difficulty. Level 1 questions require lower-order to higher-order cognitive skills to solve, while Level 4 questions require higher-order cognitive skills to solve. There are several questions in each level, but students have to solve three problems in each level before passing to the next level. If any answer is incorrect, the problem is replaced by a new question with the same difficulty. These games could challenge and encourage students to enjoyably get through the exercise although some of them are a bit difficult.
Each assignment and problem set in these exercises is accompanied by a solution and explanation as well as constructive and relevant feedback on student learning progress. Therefore, students can discover how much they have learned about NMR spectroscopy” and how well they can solve NMR problems or interpret NMR spectra. Moreover, they can identify their weakness in any concept and can review relevant sections to improve their knowledge and NMR problem-solving skills.
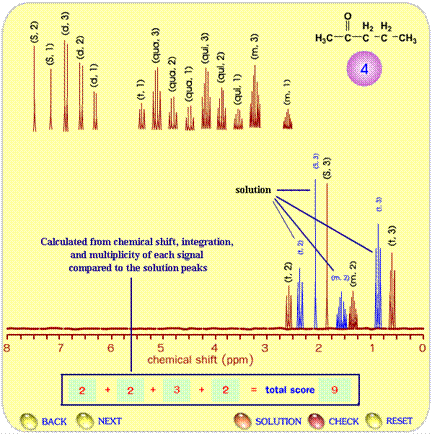
Figure 4. The scoring page for an NMR spectrum prediction test
Methodology
Research design and questions
The aim of this research was to assess the impact of the treatment (iNMR intervention) by comparing the achievement of students completingthe iNMR course to that of a control group consisting of students who completed an NMR spectroscopy course over the previous four years (2002-2005). A second aim was to compare students’ achievement before and after using the iNMR course. Therefore, the design of this study was pre-experimental (one-group pretest-posttest) which includes a pre-test measure followed by a treatment and a post-test (Creswell, 2003). When the iNMR course was implemented, following research questions were posed.
- How did the iNMR course affect students’ achievement (grade) in the Spectroscopic Methods of Chemical Identification course as compared with previous students who had no experience of using the iNMR?
- How did the iNMR course affect students’ gain in knowledge of NRM concepts as assessed by pre-test and post-test instrument?
- How did the students evaluate the iNMR course in terms of objectives and assessments, knowledge gained from the course, usability, and attitudes toward using the course?
Participants
With prior permission from the course instructors, 26 chemistry students enrolled in Spectroscopic Methods of Chemical Identification during the 1/2006 semester at the Ubon Ratchathani University volunteered to participate in this study. Right after spending 25 hours for studying Ultraviolet-Visible (UV-Vis) and Infrared (IR) spectroscopy, and Mass (MS) spectrometry in their regular class, these students were about to begin studying NMR topic for 20 hour. Data collected from students who had taken the course in the previous four years without access to the iNMR course provided a baseline against which the impact of the iNMR course could be compared.
Implementation of the iNMR course
Students in the treatment group were given both CD-ROM and URL (www.il.mahidol.ac.th/nmr) for accessing the iNMR course as a self-learning supplement for the topic of NMR spectroscopy in the Spectroscopic Methods of Chemical Identification course (three credits or three hours of lecture per week). They agreed to participate in the following steps, which took place at the same time that NMR was covered in the normal spectroscopy class.
1) Study introductory NMR concepts both in the regular class and in the iNMR course. The aim of this stage was to prepare students for the concepts of chemical shift and multiplicity, and for solving NMR problems.
2) Study the chemical shift in the regular class followed by a pre-test on chemical shift.
3) Self study of chemical shift and factors influencing chemical shift in the web-based course followed by a post-test on chemical shift.
The topics of multiplicity and NMR problem solving were studied in the same manner. After the process was completed, the students were asked to complete a attitude questionnaire to evaluate the web-based course. The iNMR course was used as a supplement to the course to be completed outside of class time and so may have encouraged students to spend more time on task. Many students may have replaced time spent on traditional materials with time spent on the iNMR course because they were provided with feedback on their understanding in the interactive course. However, the amount of time spent studying the iNMR course and the traditional course materials are not known.
Analysis of the data
Data collected from this study consists of 1) students’ pre-test and post-test scores, 2) students’ grade in the course, and 3) student evaluation of the INMR course.
1) Analysis of Students’ Pre-test and Post-Test Scores
The average pre-test and post-test scores on the concepts of chemical shift, multiplicity, and NMR problem-solving were compared (the same questions were used in each test, but in a different order) to identify improvement in content knowledge.
2) Analysis of Students’ Grade in the Course
After the final examination, students were graded on an A to F scale in which A means very good, D means poor, and F means that the student has failed the course. These data were compared to former students’ grades in the course. An independent t-test analysis was performed to identify the mean difference in course grade between the students completing the iNMR course and the former students who had not competed the iNMR course.
3) Analysis of Students’ Evaluation of the iNMR Course
The student evaluation questionnaire provided feedback on four aspects of the iNMR course: 1) students’ perception of their gain in content knowledge of NMR, 2) fulfillment of the objectives and assessments, 3) usability of the course as an online learning supplement, and 4) attitudes toward using the course. In the questionnaire, students were asked to rate their opinion of Likert-scale questions (5 is strongly agree and 1 is strongly disagree). The means of each question in each section were calculated.
Results and Discussion
Students’ Pre-test and Post-Test Scores
For the 26 students who completed the iNMR course, the average pre-test score for the concept of chemical shift was 3.96 (SD = 0.82, maximum score = 8), for multiplicity it was 4.08 (SD = 1.49, maximum score = 8), and for NMR spectrum interpretation it was 4.31 (SD = 1.19, maximum score = 8), as shown in Table 1. The average post-test score was statistically higher than the pre-test score for the concept of chemical shift by 14.88% (1.19, SD = 0.98), for multiplicity by 15.38% (1.23, SD = 0.76) for multiplicity, and NMR spectrum interpretation by 17.75% (1.42, SD = 0.95). A paired-samples T-test analysis showed that the post-test scores for each of these three concepts were statistically higher than the pre-test scores at p < 0.001. The significant increases indicate that the iNMR course was effective in helping these students to improve their understanding of the concepts of chemical shift, multiplicity, and NMR spectrum interpretation.
Table 1. Students’ pre-test and post-test scores for chemical shift, multiplicity, and NMR spectrum interpretation concepts (n = 26)
Concept |
Pre-test |
Post-test |
Gain |
t-test |
mean |
SD |
mean |
SD |
mean |
SD |
t |
p |
Chemical shift |
3.96 |
0.82 |
5.15 |
0.92 |
1.19 |
0.98 |
6.200 |
< 0.001 |
Multiplicity |
4.08 |
1.49 |
5.31 |
1.16 |
1.23 |
0.76 |
8.208 |
< 0.001 |
spectrum interpretation |
4.31 |
1.19 |
5.73 |
1.19 |
1.42 |
0.95 |
7.675 |
< 0.001 |
Total |
12.35 |
1.98 |
16.19 |
2.17 |
3.85 |
1.38 |
14.245 |
< 0.001 |
Students’ Grades in the Course
Since the score on NMR was about 44% of the entire course, improvement in the NMR score could increase the course grades in the Spectroscopic Methods of Chemical Identification course. The average course grade during the years 2002-2006 are shown in Table 2. The result from an independent t-test analysis indicates that the average course grade of students experiencing the iNMR course (mean 2.73, SD 1.06) is significantly higher than the average course grade of students who experienced only the traditional approach in each year during 2002-2005 at a p value less than 0.03. In short, the current regular NMR course used in conjunction with the supplemental iNMR course added significantly improvement to the courses over the previous 4 years (2002-2005). In other word, the iNMR course can help students improve their NMR learning as their average course grade was higher than the control.
Table 2. Students’ average course grade in the Spectroscopic Methods of Chemical Identification course during 2002 to 2006
Year |
No. of students |
Learning approach |
Course grade |
|
t-test (compare to iNMR ) |
mean |
SD |
|
t |
p |
2002 |
33 |
traditional |
2.18 |
0.87 |
|
2.19 |
0.03 |
2003 |
25 |
traditional |
2.10 |
0.90 |
|
2.28 |
0.03 |
2004 |
26 |
traditional |
2.07 |
1.00 |
|
2.31 |
0.02 |
2005 |
28 |
traditional |
2.14 |
0.88 |
|
2.50 |
0.02 |
2006 |
26 |
iNMR |
2.73 |
1.06 |
|
|
|
Student Evaluation of the iNMR course
To strengthen the quantitative implications of this study, four aspects of the iNMR course were evaluated. On the questionnaire completed by the students, the means for each statement in each section were in the range of 3.5-4.5, which indicates that most of the students have positive agreement with the statements (Table 3).
Table 3. Examples of criteria for student evaluation of the iNMR course (5 is “strongly agree” and 1 is “strongly disagree”)
Item |
Mean |
SD |
|
Section A: Objectives and assessments |
|
- The INMR course objectives were clearly stated.
|
3.92 |
0.63 |
|
- The topics presented in the INMR course were relevant to me.
|
3.92 |
0.63 |
|
- My expectations were met.
|
3.65 |
0.63 |
|
- The exercises matched well with the content of the course.
|
3.58 |
0.70 |
|
- The self-evaluation was useful in the course.
|
3.77 |
0.51 |
|
Section B: Knowledge gained from the INMR course |
|
- When finished studying the web course, I was able to do assignments.
|
3.58 |
0.72 |
|
- English used in the INMR course was easy to understand.
|
3.58 |
0.84 |
|
- The INMR course can improve my understanding of NMR, especially chemical shift, Multiplicity and NMR spectrum interpretation concepts.
|
3.54 |
0.69 |
|
- After studying the web course, I was able to interpret NMR spectra more quickly.
|
3.58 |
0.65 |
|
Section C: Usability of the INMR course as an online course |
|
- The INMR course was easy to access.
|
3.73 |
0.60 |
|
- The INMR course was easy to navigate and follow.
|
3.73 |
0.64 |
|
- Animations in the course was easy to understand
|
3.65 |
0.95 |
|
- Assignments in the course drew my interest to work through them.
|
3.65 |
0.76 |
|
- The INMR course can display each page quickly.
|
3.69 |
0.81 |
- The order of contents in the INMR course was appropriate for self-learning.
|
3.65 |
0.71 |
- The INMR course was appropriate to use as an iNMR course.
|
3.88 |
0.91 |
Section D: Attitudes toward using the iNMR course |
- The INMR course was a good option for studying NMR spectroscopy.
|
4.27 |
0.49 |
- The INMR course was more interactive than a textbook.
|
4.19 |
0.69 |
- I prefer learning with the INMR course rather than a textbook.
|
4.00 |
0.80 |
- I prefer studying in a regular class with INMR course rather than a regular class only.
|
4.00 |
0.69 |
Considering the objectives and assessments of the course, students agreed that the course stated objectives clearly, presented relevant topics, and contained exercises with sufficient and relevant feedback. In terms of gain in content knowledge, students agreed that the course helped them to improve their understanding of chemical shift, multiplicity, and NMR spectrum interpretation concepts. They also agreed that after studying the course, they were able to work through assignments and interpret NMR spectra faster and more accurately. In term of usability of the online course, they agreed that this course was appropriate for online learning, easy to navigate and follow, and appropriate for self-learning. On attitudinal items most students agreed that the course was a good way to study NMR spectroscopy. They preferred studying NMR spectroscopy in a regular class accompanied with the course to studying only in a regular class.
The students also provided information about what they liked most in the course; for example,
I like animations because they help me to understand some difficult concepts.
I like the variety of assignments. It is fun to try.
I like the assignments provided with solutions and relevant feedback.
The students also gave suggestions to improve the course, for example;
There should be a Thai version because my English is poor.
It will be better to have more assignments and exercises because it’s good to practice on the assignments.
It will help a lot if more animations on some difficult topics are included.
It will be complete if the UV-Vis, IR, and Mass interactive website can be constructed.
Modification to the iNMR course
The iNMR course was modified according to the experts’ and students’ comments and suggestions. English language in the course was simplified for students for whom English is a second language. More animations were developed to explain some intangible concepts. More exercises and assignments were included to give students more opportunities to practice applying their knowledge of NMR. The course is now available both in English ( http://www.chem.sci.ubu.ac.th/inmr/) and in Thai (http://www.chem.sci.ubu.ac.th/inmr_th/).
Conclusion and Implications
The study findings show that students who used the interactive course as a supplement to a traditional class can significantly improve their conceptual understanding of the concepts of chemical shift and multiplicity, and their NMR problem solving (or spectra interpretation) skills. This improvement may be due to the fact that the iNMR course promoted constructivist learning, in which students are provided access to rich sources of information, meaningful interactions with content are encouraged (Chin & Williams, 2006; Wilson & Lowry, 2000), and students are allowed to take control of their learning (Lefoe, 1998). Students who completed the interactive course achieved higher course grades than students in previous years; however, it is not known whether this effect was due to increased time on task. Students used the interactive course on their own time and could spend as much or as little time as they wanted, just as they did with other supplemental materials such as the course textbook.
The students “agreed” that the iNMR course stated objectives clearly, presented relevant topics, and contained exercises with sufficient and relevant feedback. The course helped them to improve their understanding of chemical shift, multiplicity, and NMR spectrum interpretation concepts. Therefore, this course was appropriate for use as self-studying or online-learning tool. In addition, the students suggested that this course will also be useful for students who have passed an NMR spectroscopy class and want to review and practice NMR spectroscopy.
Based on the study findings, chemistry instructors could consider using the interactive course to help students develop their NMR understanding. To effectively implement the interactive course, instructors should consider setting the learning in a meaningful context and using a problem-based learning approach (Renkl, Mandl & Gruber, 1993; Savin-Baden, 2000). They may want to provide class time for using the interactive course, and search for student alternative conceptions and deal with them in class (Camacho & Good, 1989). In addition, instructors should use authentic assessments to see the progression in students’ NMR understanding and to plan for development of their learning ( Mueller, 2005).
Further Studies
A limitation encountered in the study was that some students have problems with the English language, so they had difficulty understanding the English text. Although animations show the concepts clearly, some students still preferred Thai text descriptions to English. Since the iNMR course is now available in Thai, the effect of iNMR course on student NMR problem solving will be investigated. In addition, protocol of how low- and middle- achievement students solve NMR problem will be discussed.
Acknowledgement
We thank the Organic Chemistry Laboratory II instructors and students during the 1/2006 semester at Ubon Ratchathani University for their enthusiastic participation in this study. This study was partial funded by the NSF Award 0440103: Design Principles for Effective Molecular Animations and by the Higher Education Commission of Thailand. Special thanks to Professor Loretta L. Jones at University of Northern Colorado for her correction of grammar errors.
References
Bodner, G.M., Klobuchar, M., & Geelan, D. (2001). The many forms of constructivism. Journal of Chemical Education , 78, 1107.
Camacho, M., & Good, R. (1989). Problem solving and chemical equilibrium: Successful versus unsuccessful performance. Journal of Research in Science Teaching, 32, 911-927.
Chin , S.T.W. & Williams, J.B. (2006). A theoretical framework for effective online course design. MERLOT Journal of Online Learning and Teaching, 2, 12-21.
Creswell, J. (2003). Research design: Qualitative, quantitative, and mixed methods approaches (2nd ed.). California: Sage publication.
Dorneich, M.C., & Jones, P.M. (1997). Supporting apprenticeship learning of NMR spectroscopy in a collaborative, web-based learning environment. Retrieved on 10 October 2008. Available at http://citeseerx.ist.psu.edu
Glasersfeld, E. von. (1992).Constructivism revisited: A reply to Sutchings. Science and Education, 1(4),379-384.
Lefoe, G. (1998). Creating constructivist learning environments on the web: the challenge in higher education. Proceedings from ASCILITE’ 98, The Australian Society of Computer in Learning in Tertiary Education Conference, University of Wollongong, Australia.
McKimm, J., Jollie, C., & Cantillon, P. (2003). ABC of learning and teaching: Web based learning. British Medical Journal, 326, 870-873.
Merlic, C.A., & Fam, B.C. (1997). WebSpectra: Problems in NMR and IR spectroscopy. Retrieved March 20, 2008, from http://www.chem.ucla.edu/~webspectra/
Mueller, J. (2005). The authentic assessment toolbox: Enhancing student learning through online faculty development. MERLOT Journal of Online Learning and Teaching, 1.
Nussbaum, J. (1989). Classroom conceptual change: Philosophical perspectives. International Journal of Science Education, 11(5), 530-540.
Renkl, A., Mandl H., & Gruber H. (1996). Inert knowledge: analyses and remedies. Educational Psychology, 31, 115-121.
Reusch, W. (1999) . Nuclear magnetic resonance spectroscopy. In Virtual Textbook of Organic Chemistry (Spectroscopy) . Retrieved February 10, 2008, from http://www.cem.msu.edu/~reusch/VirtualText/Spectrpy/nmr/nmr1.htm#nmr1/
Savin-Baden, M. (2000), Problem-based learning in higher education: untold stories, UK: Mcgraw-Hill.
Smith, B.D., Boggess, B., & Zajicek, J. (1998). Organic structure elucidation: A workbook of unknowns. Retrieved February 13, 2008, from http://www.nd.edu/~smithgrp/structure/workbook.html/
Solomons, T.W.G., & Fryhle, C.B. (2003). Organic chemistry (8 th ed.). New York: John Wiley and Sons.
Tsai, C. (2000). Relationships between student science epistemological beliefs and perceptions of constructivist learning environments. Educational Research, 42(2), 93-205.
Wilson , B., & Lowry, M. (2000). Constructivist learning on the web. New Directions for Adult and Continuing Education, 88, 79-88.
Young, P.R. (1997). Proton and carbon NMR. In Organic Chemistry OnLine (Spectroscopy) . Retrieved February 12, 2008, from http://www.chem.uic.edu/web1/OCOL-II/WIN/HOME.HTM/
Zoller, U., & Pushkin, D. (2007). Matching higher-order cognitive skills (HOCS) promotion goals with problem-based laboratory practice in. a freshman organic chemistry course, Chemistry Education Research and Practice,8, 153-171. |